Biowaivers are a means of waiving bioequivalence studies in humans.
Although, in theory, simultaneous dossier submissions can greatly streamline the drug approval process, in practice, worldwide drug marketing approvals may be delayed due to region-specific regulatory requirements or dissimilar regulatory review outcomes from agencies of different jurisdictions. As per established published regulations, regulatory agencies are tasked with ensuring the quality, safety, and efficacy of all medications that they approve for marketing within their respective countries.
Biowaivers
Thus, designing a regulatory strategy that would meet the diverse needs of regulators worldwide is a major challenge for drug developers who seek global markets. One approach for which regulators have made great strides toward international harmonization is the application of the Biopharmaceutics Classification System (BCS).
The BCS is an important tool for waiving the regulatory requirement for in vivo bioavailability (BA) and/or bioequivalence (BE) studies in both new and generic drug development. This review will explain the BCS-based biowaiver process and its role in drug development, describe how the BCS biowaiver approach evolved from its inception to its present state, and summarize the current state of the BCS biowaiver implementation in several jurisdictions throughout the world.
Regulatory agencies have long recognized that, for some drug products, in vivo BA/BE may be self-evident and, as such, can waive the requirement for in vivo evidence in certain circumstances (to grant a biowaiver). The use of biowaivers is in the spirit of avoiding unnecessary human testing whenever possible and facilitates access to drugs in jurisdictions such as the USA, EU, Canada, Australia, and also emerging countries.
Application of the BCS in waiving BA/BE requirements is based on premises that
- if two immediate-release (IR) drug formulations/products behave as oral solutions within the GI tract due to high solubility and rapid dissolution,
- no precipitation occurs in the GI tract once the API is dissolved
- the two IR formulations have the same in vivo dissolution profile under all intestinal luminal conditions, then they should have the same rate and extent of absorption, and therefore be bioequivalent.
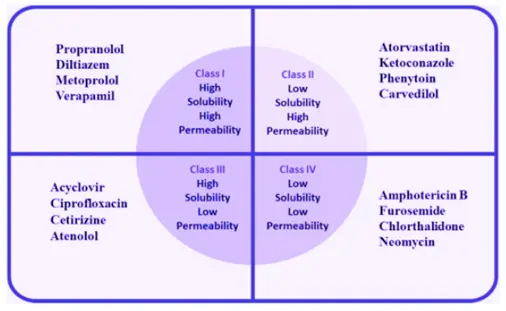
Another premise underlying the development of the BCS is the recognition that the two key factors governing drug absorption are aqueous solubility and intestinal permeability. Thus, the BCS is a scientific framework for classifying drug substances based on their aqueous solubility and intestinal permeability. As such, the BCS designates all drugs as belonging to one of four classes:
- Class I (high solubility, high permeability)
- Class II (low solubility, high permeability)
- Class III (high solubility, low permeability)
- Class IV (low solubility, low permeability)
Ability to waive in vivo BE requirements using the BCS biowaiver approach can be pivotal to a successful regulatory submission, because demonstrating that two drug formulations/products are bioequivalent is an essential drug approval requirement for both innovator and generic drug products. Two drug formulations/products are deemed bioequivalent when they do not differ significantly in the rate and extent to which the active ingredient or active moiety becomes available at the site of action when the two formulations/products are given at the same molar dose under similar experimental conditions in an appropriately designed study.
In generic drug development, demonstration of BE between the generic and its corresponding reference product is required for approval. BE evidence is also required when commercialized drug products (whether new or generic) undergo certain types of scale-up or postapproval changes. Thus, in any of these regulatory situations where BE studies are deemed necessary for an IR solid oral dosage form, the potential exists for a BCS-based biowaiver, provided that the appropriate BCS designation criteria are met.
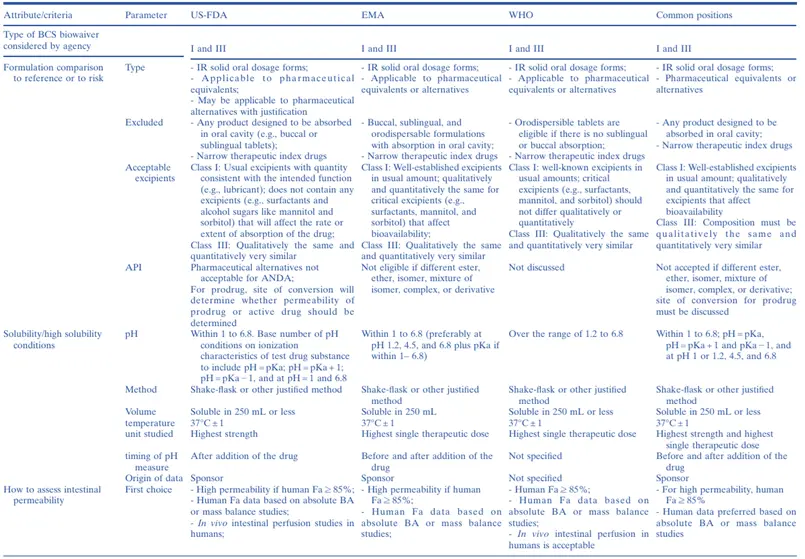
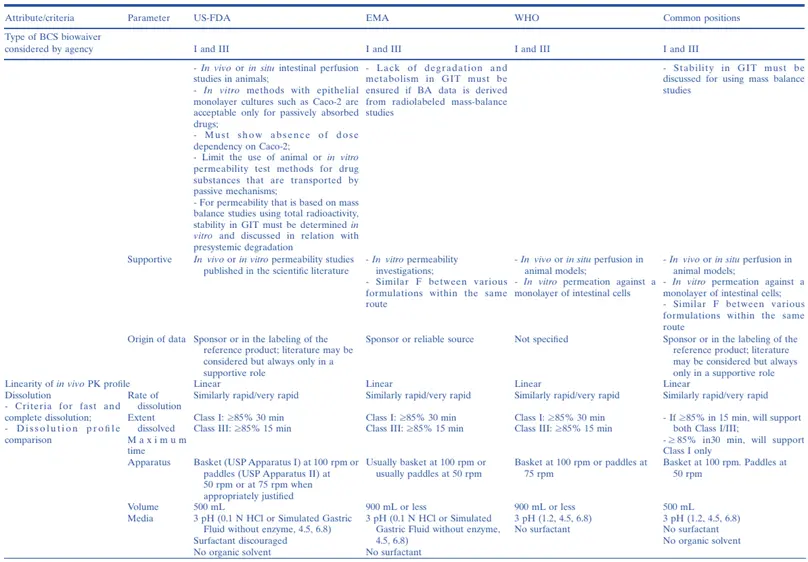
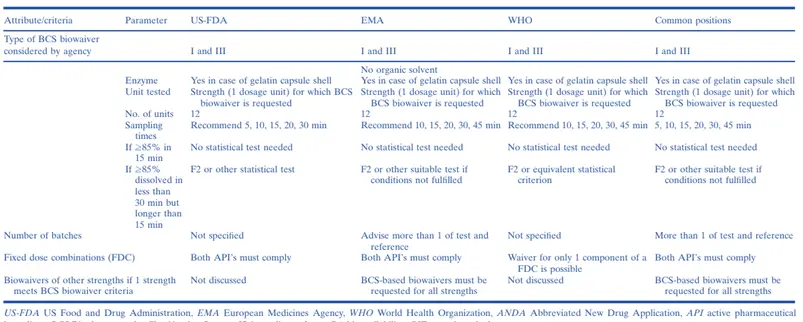
The first regulatory guidances encouraging the use of the BCS for biowaivers of Class I immediate-release (IR) solid oral dosage forms were issued by the US Food and Drug Administration (US-FDA) in 2000 and by the European Medicines Agency (EMA) in 2001. Later, the World Health Organization (WHO) and EMA published guidelines allowing the granting of BCS biowaivers for both Class I and Class III drugs. Initially, the WHO also considered granting biowaivers for some drugs of weak acids in Class II but presently grants biowaivers for only Class I and Class III drugs. In May 2015, the US-FDA revised its BCS Guidance to expand the biowaiver provision to Class III drugs. Subsequently, in July 2015, the US-FDA posted a new draft Guidance for Industry which provided recommendations for in vitro dissolution testing and specification criteria for immediate-release solid oral dosage forms containing BCS Class I and Class III drugs. The EMA and US-FDA publish individual BE or product-specific guidances advocating the use of BCS biowaivers, and the International Pharmaceutical Federation (FIP) has published 44 monographs on the subject. Presently, many emerging markets are implementing the BCS biowaiver approach based on jurisdiction-specific guidelines or the WHO guidelines.
Thus, the BCS is now widely established in the academic, industrial, and regulatory world for waiving the regulatory requirements of conducting comparative bioavailability studies to demonstrate in vivo BA/BE of immediate-release (IR) solid oral dosage forms containing BCS Class I and Class III drugs. However, although great advances have been made recently in harmonizing some aspects of BCS biowaiver implementation among major regulatory jurisdictions, there remain a number of dissimilarities among them (notably, in Japan, the BCS biowaiver has not been implemented at all), presenting a challenge for innovator and generic companies that seek to utilize a BCS biowaiver approach for global registration of a drug product.
User Also read: Tablet Scoring in Pharmaceuticals

