Learn the ICH Q2(R1) guidelines for Validation of Analytical Procedures in the Pharmaceutical Industry
Understanding ICH Q2(R1): Validation of Analytical Procedures in the Pharmaceutical Industry
What Is ICH and Its Global Mission?
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is a globally recognized body that unites regulatory authorities and pharmaceutical industry stakeholders. Its primary mission is to promote international harmonization in the development and registration of pharmaceuticals, ensuring that safe, effective, and high-quality medicines reach the global market efficiently and consistently.
By establishing unified standards and practices, ICH helps eliminate duplicative testing, reduces development costs, and accelerates access to life-saving medications across different regions.
Key Harmonization Achievements in Pharmaceutical Quality
One of the most impactful contributions of ICH in the quality domain includes the development of guidelines for:
- Stability studies of pharmaceutical products
- Impurity testing and defining critical threshold levels
- Adoption of Good Manufacturing Practice (GMP)-based risk management models
These efforts support a more flexible, science-based regulatory framework, fostering innovation while safeguarding patient safety.
ICH Q2(R1): Validation of Analytical Procedures – Overview
Among the most critical ICH guidelines is ICH Q2(R1), which focuses on the validation of analytical procedures. This guideline is a collaborative output applicable to regulatory submissions in the European Union (EC), Japan, and the United States.
Rather than prescribing exact validation methods, ICH Q2(R1) offers a standardized terminology and framework to harmonize analytical validation across different regulatory bodies. It ensures that analytical procedures are suitable for their intended use, whether in drug substance or drug product testing.
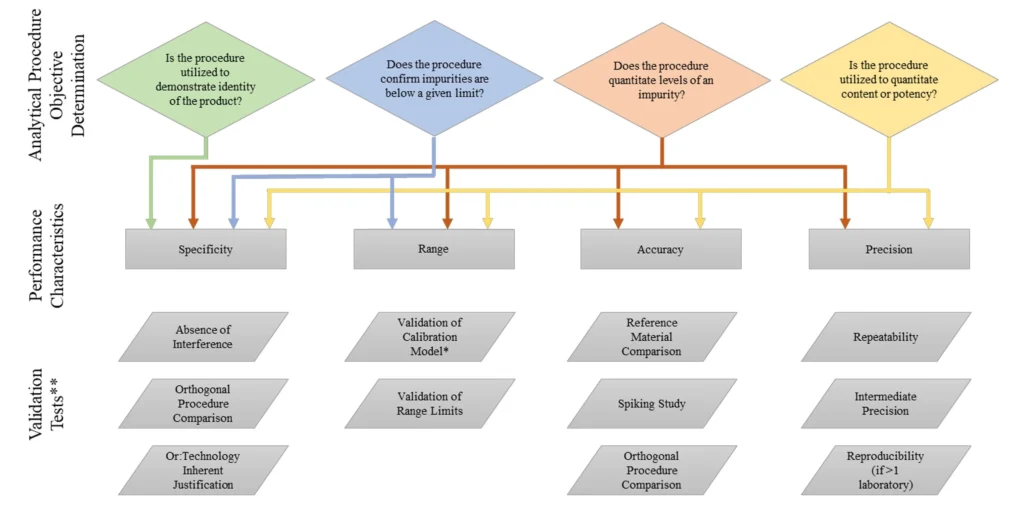
Objectives of Analytical Method Validation
The primary objective of analytical procedure validation is to demonstrate reliability, accuracy, and reproducibility in measuring the intended analyte within a pharmaceutical product. The validation parameters are selected based on the purpose of the test, such as identification, impurity testing, or assay quantification.
A tabular summary of relevant characteristics is provided in the guideline to match validation parameters with their appropriate application.
Types of Analytical Procedures Covered Under ICH Q2(R1)
The guideline emphasizes the validation of four major types of analytical tests:
- Identification Tests
Designed to confirm the identity of an analyte by comparing it to a reference standard (e.g., spectrum, chromatography, chemical reactivity). - Quantitative Tests for Impurities
These determine the actual content of impurities and require high sensitivity and accuracy. - Limit Tests for Impurity Control
Aim to confirm whether impurities fall within acceptable limits. These differ from quantitative methods in validation parameters. - Assay Procedures
Used to quantitatively measure the active pharmaceutical ingredient (API) or other significant components in a sample.
While other analytical techniques like dissolution tests or particle size analysis are crucial, they are not addressed in this specific version of the guideline but may be covered in future updates.
Core Validation Characteristics
Each type of analytical procedure must be evaluated for specific validation parameters, including:
- Accuracy
- Precision (Repeatability and Intermediate Precision)
- Specificity
- Detection Limit (LOD)
- Quantitation Limit (LOQ)
- Linearity
- Range
Robustness is another important attribute, although not always listed in summary tables, and should be assessed during method development.
When Is Revalidation Required?
Revalidation of analytical methods may be necessary under the following conditions:
- Modifications in the drug substance synthesis
- Changes in formulation composition
- Alterations to the analytical methodology itself
The extent of revalidation will depend on the magnitude and nature of the change. A risk-based approach is often used to determine the appropriate validation effort.
Final Thoughts: Advancing Pharmaceutical Quality Through Harmonization
ICH has significantly transformed the landscape of pharmaceutical development by introducing globally accepted standards. The Q2(R1) guideline on analytical method validation ensures that pharmaceutical companies consistently produce reliable data, meet regulatory expectations, and most importantly, uphold patient safety.
As the pharmaceutical industry continues to innovate, harmonized validation practices remain essential for efficient drug approval processes and cross-border regulatory compliance.

